
-
Whitepaper
Medical Devices
Developing and Maintaining a Quality Management System for IVDs
This paper primarily examines the QMS requirements in the new In Vitro Diagnostic Device Regulation (IVDR) 2017/7462.
Article 10 (8) of the IVDR establishes a number of requirements.
This paper primarily examines the QMS requirements
-
QMS Basics
-
Strategy for regulatory compliance
-
Risk management
-
Performance evaluation and postmarket surveillance
-
Unique Device Identification (UDI)
-
Continuous improvement activities
BSI is your partner for regulatory compliance
Discover our medical device standards and certification services below.
-
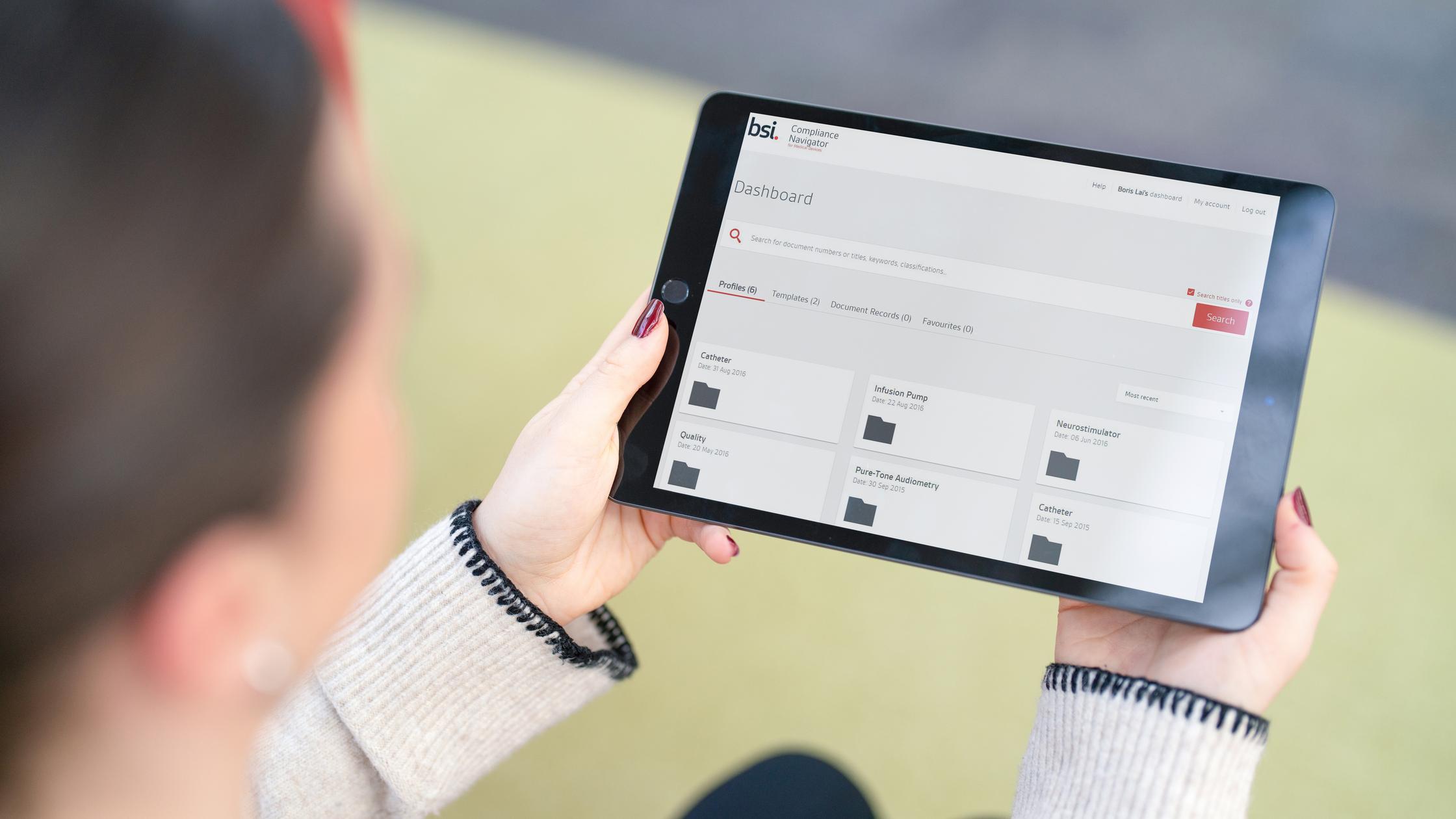
Compliance Navigator
A digital platform transforming how medical device and IVD device manufacturers manage their regulatory documents to save time and reduce risk.
Find out more
-

BSI Knowledge
Deliver quality, improve safety and stay compliant with access to BSI’s healthcare standards. Embed best practice with a company-wide subscription.
Find out more
-
Medical Devices
Our mission is to ensure patient safety whilst supporting timely market access to medical technology in a sustainable manner.
Find out more
Speak to the Compliance Navigator team today
Transform your document management process and stay ahead of medtech regulatory updates. Talk with our experts today and arrange a free trial.


